This story was used with permission from the University of Minnesota.
A molecular band-aid
A bleeding heart is a metaphor.
A leaking one is a common, and often deadly, reality.
In conditions from Duchenne muscular dystrophy to heart attack and heart failure, leaky heart cells lose proteins vital to long-term survival. For University of Minnesota heart researcher Joseph Metzger, fixing these leaks is a prime concern.
He is part of a U team that has built and used molecules akin to plastic as “molecular band-aids” to repair tears in the cell membranes that enclose muscle cells, keeping those vital proteins inside. Collectively, the patches are known as poloxamers.
“The FDA has approved one form of poloxamer for clinical trials with boys who have Duchenne muscular dystrophy,” says Metzger, head of the Department of Integrative Biology and Physiology. “But [we still must secure] funds for the trials.”
Metzger’s colleagues are Frank Bates, head of the Department of Chemical Engineering and Materials Science, and cardiologist Demetri Yannopoulos, an assistant professor of medicine.
How hearts leak
In Duchenne muscular dystrophy (DMD), a child—almost always a boy—is born with one of many mutations that lead to the absence of a large protein called dystrophin.
“Dystrophin is like a shock absorber. It dissipates the force from contraction,” Metzger explains.
“Without it, a muscle gets battered. The cell membrane is rendered highly susceptible to damage and unstable. We don’t know exactly where the membrane becomes unstable, but we can observe big proteins, including enzymes, leaking out.”
Following a heart attack and in cases of heart failure, damaged and/or aging heart cells can also become leaky. An acquired deficiency or alteration in dystrophin, Metzger points out, is one of many paths to dysfunction in acquired heart diseases, but in DMD it’s the lesion, affecting both heart and skeletal muscles.
Certain steroids can help improve the function of limb muscles in DMD patients, and other treatments can help the diaphragm and other respiratory muscles.
“These don’t cure DMD, but now boys can live into their twenties, not just early teens,” says Metzger. “It’s a cruel irony that if you [help] other muscles, the heart muscle is still weak.” In fact, he notes, many DMD patients die from heart failure.
Dystrophin can’t be replaced, but the molecular band-aids Metzger and his colleagues have developed may take over some of its function of keeping a cell from rupturing during stressful contractions. Here’s how:
‘Like the little Dutch boy’
The molecular band-aid comes about its name honestly. It is basically a molecular chain, or strip, with two identical “sticky” end pieces flanking a central piece that “covers the wound.”
The diagram below shows the FDA-approved poloxamer. It consists of two end pieces, each a string of 80 molecules of polyethylene oxide (blue), and a central string of 27 molecules of polypropylene oxide (red). It is thought to drape itself over a cell membrane, seen in cross section with the membrane’s outer surface in blue on top.
The end pieces are chemically attracted to the outer surface and anchor the band-aid. But the central piece is chemically drawn to the inner part of the membrane (yellow), or to the cell contents, which it reaches by dipping down through the breach. Either way, the poloxamer is hypothesized to plug the membrane tear “like the little Dutch boy sticking his finger in the dike,” Metzger says.
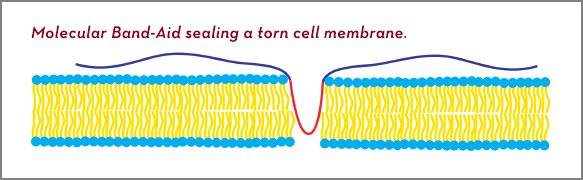 Diagram of a molecular band-aid plugging a hole in a cell membrane.
Diagram of a molecular band-aid plugging a hole in a cell membrane.
“Frank [Bates] makes different lengths or varies the composition and architecture of the band-aids,” he adds. “We’re trying to optimize them for a range of diseases, for example, heart failure and DMD.”
Encouraging results
For his part, Yannopoulos works to prevent heart damage that occurs when a doctor reopens a clogged coronary artery after a heart attack. Lack of oxygen and nutrients during an attack causes a condition called rigor (as in rigor mortis), which primes muscle cells to respond to the restoration of oxygen with inappropriate contractions and, often, more damage to cell membranes; this is called reperfusion injury.
But in an animal model of a heart attack and restoration of blood flow, the researchers found that treatment with a poloxamer led to much less reperfusion injury.
Molecular band-aids, says Metzger, have the advantages of being chemically inert and don’t appear to trigger an immune response. To be effective, however, they would have to be injected directly into a coronary artery as it is reopened, or on a quasi-daily basis for chronic conditions.
Compared to living with heart failure or the ravages of Duchenne muscular dystrophy, that sounds like a good deal.
Joseph Metzger holds the Maurice B. Visscher Endowed Land-Grant Chair in Physiology. Read related stories on molecular band-aids and a “guardian angel” protein for heart patients, a calcium sponge for diastolic heart failure, and research to improve CPR success.
Contact the writer at morri029@umn.edu
